Green Light Triggers Antibiotic Activation Precisely Where Needed
Green Light Triggers Antibiotic Activation Precisely Where Needed
In the ongoing battle against antibiotic resistance, an innovative approach harnessing the power of light has emerged, promising a transformative paradigm in bacterial infection treatment. Researchers have engineered a novel version of penicillin that remains inactive until exposed to green light, enabling unprecedented spatial and temporal control over antibiotic activation. This breakthrough not only curtails the indiscriminate use of antibiotics but also offers a targeted strategy that could significantly reduce the emergence of resistant bacteria strains in the environment.
Traditional antibiotic therapies suffer from systemic distribution, where active drugs circulate throughout the body, inevitably reaching unintended sites and sometimes contributing to environmental contamination. The lingering residues of these antibiotics in wastewater facilitate the proliferation of antimicrobial-resistant bacteria, posing a grave public health threat. By redesigning penicillin with a light-activatable molecular “cage,” scientists have skillfully circumvented this issue. The modified drug remains chemically inert until illuminated, releasing its bactericidal potency only where necessary.
This method leverages a coumarin-based photolabile protecting group attached to the penicillin molecule, effectively “caging” the antibiotic’s active moiety. Upon illumination with green light, this protective group undergoes cleavage, liberating functional penicillin capable of disrupting bacterial cell wall synthesis. Unlike previous light-activation systems that required high-energy ultraviolet or blue light, which have limited tissue penetration and can damage healthy cells, this system’s use of green light represents a safer and more clinically relevant wavelength.
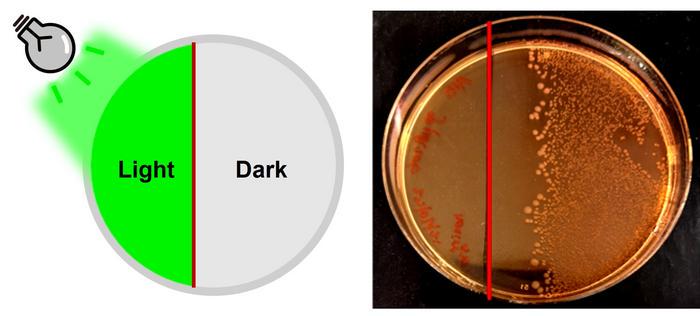
Experimental validation began with cultured bacterial strains, including Escherichia coli and Staphylococcus epidermidis. When exposed to green light in the presence of the modified penicillin, bacterial growth was selectively inhibited, demonstrating precise photocontrol over antibiotic activity. Remarkably, a defined boundary emerged where illuminated areas exhibited sterilization, whereas non-illuminated regions sustained bacterial proliferation. This spatial selectivity holds profound implications for localized infection management where systemic exposure is undesirable.
Moving beyond in vitro assessments, the researchers turned to an in vivo model—the larvae of the wax moth Galleria mellonella, which serves as a surrogate for human immune response owing to its conserved innate immunity mechanisms. Larvae infected with Staphylococcus aureus were treated with the light-activated penicillin followed by targeted green light therapy. This treatment led to a marked improvement in survival rates, doubling those of untreated controls. This milestone underscores the potential translational value of this technology in living systems.
Such photopharmacological approaches envision a future where clinicians wield spatially and temporally precise control over therapeutic interventions. By exploiting the properties of different light wavelengths, it becomes conceivable to activate multiple drugs independently within the same organism, each responsive to a unique color of light. This layered control could revolutionize treatment regimens for multifaceted infections or polymicrobial biofilms which currently defy conventional antibiotics.
The development of green-light-responsive antibiotics also addresses critical pharmacological challenges. Green light penetrates biological tissues more effectively than ultraviolet or blue wavelengths, minimizing damage and enhancing treatment depth. Additionally, the reversible activation mechanism reduces systemic toxicity, as the antibiotic remains inactive outside the irradiation zone, preserving beneficial microbiota and reducing side effects typically associated with broad-spectrum agents.
The synthesis of these green-light-activatable penicillin derivatives involved sophisticated organic chemistry techniques to conjugate coumarin molecules to the β-lactam ring of penicillin. This chemical modification transiently masks the antibiotic’s active site, preventing premature activity. Precise photolysis kinetics ensured that upon illumination, the protective group dissociated rapidly and efficiently, restoring penicillin’s antibacterial function. Optimization of light exposure parameters balanced activation efficacy with minimal photodamage.
Biofilm formation, a major contributor to chronic infections, was also significantly mitigated through this approach. The green light-triggered penicillin inhibited Staphylococcus epidermidis biofilm development, a notoriously resilient bacterial community structure that complicates treatment. Disrupting biofilms locally via light-activated antibiotics introduces a new arsenal against persistent infections, particularly device-associated or wound-related biofilms.
Looking forward, this research paves the way for multi-modal therapies combining photopharmacology with existing clinical practices. Integration with fiber-optic light delivery systems or wearable phototherapy devices could allow real-time control of antibiotic activity within deep tissues or localized sites, enhancing patient compliance and therapeutic outcomes. Furthermore, iterative design could extend this activation strategy to other clinically relevant antibiotics, broadening the scope of precision antimicrobial therapy.
While challenges remain, including light penetration limitations in human tissues and ensuring uniform drug distribution prior to activation, the demonstration of a living organism model efficacy is a significant leap. Continued interdisciplinary collaboration spanning medicinal chemistry, microbiology, and clinical science holds promise for translating this innovative concept into therapeutic reality.
Altogether, this green-light-activated penicillin paradigm introduces a powerful tool against the rising tide of antibiotic resistance. By confining antibiotic activity to targeted zones and reducing off-target exposure, it aligns with global health priorities aiming to preserve antibiotic efficacy and protect ecosystems from pharmaceutical contamination. The implications extend beyond medicine, heralding a new era of light-controlled drugs with enhanced safety, effectiveness, and adaptability.
Subject of Research: Development of green-light-activatable penicillin for precise, localized control of bacterial growth and infection treatment.
Article Title: Green-Light-Activatable Penicillin for Light-Dependent Spatial Control of Bacterial Growth, Biofilm Formation, and In Vivo Infection Treatment
News Publication Date: 11-Jun-2025
References:
Schulte, A., Schoenmakers, J., et al. “Green-Light-Activatable Penicillin for Light-Dependent Spatial Control of Bacterial Growth, Biofilm Formation, and In Vivo Infection Treatment.” ACS Central Science (2025). DOI: 10.1021/acscentsci.5c00437
Image Credits: Adapted from ACS Central Science 2025, DOI: 10.1021/acscentsci.5c00437
Keywords
Chemistry | Health and medicine | Antibiotics | Infectious diseases
Green Seaweed Overtakes Seagrass as Slugs Emerge as New Threats
Next PostNew JNCCN Study Highlights Telehealth’s Role in Bridging Geographic and Resource Barriers in Global Cancer Care
Breakthrough in Two-Photon Upconversion: 2D Excitons Power Giant Boost in Doubly-Resonant Plasmonic Nanocavities